

Industrial Process for Producing L-Lysine by Fermentation
Sep 12, 2025
When I speak with clients from the feed and fermentation industries, one of the most frequent questions I hear is: “How do companies actually produce L-lysine on an industrial scale?” It sounds like a straightforward question, but the process behind it is both fascinating and highly technical. L-lysine is one of the most important essential amino acids for humans and animals, and its large-scale production is a cornerstone of the global nutrition industry.
The industrial production of L-lysine by fermentation is based on using genetically optimized microbes such as Corynebacterium glutamicum cultivated in large bioreactors. These microorganisms convert renewable raw materials like glucose, starch hydrolysates, or molasses into L-lysine under tightly controlled aerobic conditions. The resulting broth is purified into crystalline L-lysine or L-lysine hydrochloride for use in animal feed, food supplements, and pharmaceuticals.
In this article, I will guide you through the main stages of the fermentation process, highlight why L-lysine matters, and explain related questions about amino acid production and terminology. If you are planning a lysine production project, or simply want to better understand the value chain, this step-by-step overview will give you practical insight.
How is L-Lysine Produced Industrially by Fermentation?
The production process has evolved over decades, becoming highly efficient and cost-effective. Let me break down the key stages so you can see the logic and flow behind them.
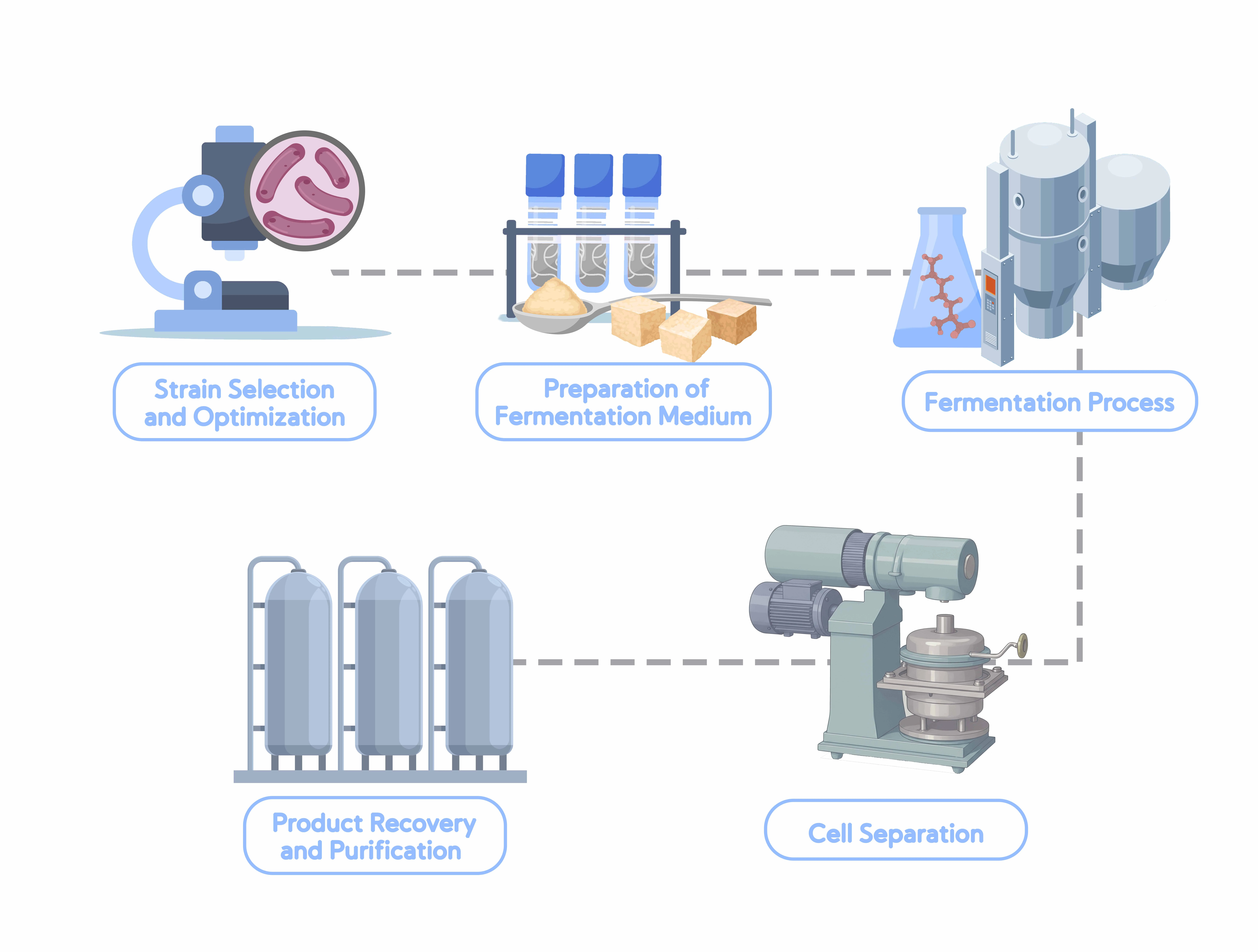
1. Strain Selection and Optimization
Everything starts with the microorganism. Industrial producers commonly use genetically engineered strains of Corynebacterium glutamicum or Escherichia coli. These strains are designed to:
◼️ Resist feedback inhibition.
◼️ Redirect metabolic pathways toward lysine accumulation.
◼️ Tolerate higher concentrations of the amino acid.
| Step | Organism Used | Key Feature |
| Strain Selection | Corynebacterium glutamicum, E. coli | High yield and stability |
| Genetic Optimization | Resistant to feedback inhibition | Improves lysine accumulation |
| Fermentation Robustness | High tolerance | Suitable for long cycles |
Without the right strain, even the most advanced plant cannot achieve commercial efficiency.
2. Preparation of Fermentation Medium
The microbes need food. The medium provides all essential nutrients:
◼️ Carbon Source: Glucose, starch hydrolysates, or molasses (around 10% concentration).
◼️ Nitrogen Source: Ammonium salts, urea, or ammonia.
◼️ Minerals & Trace Elements: Phosphates, magnesium, iron, and others.
◼️ Growth Factors: Vitamins or precursors added as boosters.
pH is adjusted (usually with ammonia) to around neutral levels, which is crucial for stable growth and enzyme activity.
3. Fermentation Process
This is where the real transformation happens. Microbes convert sugars and nitrogen into L-lysine.
Core conditions:
◼️ Aerobic environment with high oxygen transfer.
◼️ Temperature maintained at 37–40°C.
◼️ pH kept around 7.0.
◼️ Strong mixing to avoid gradients.
Fermentation modes vary:
◼️ Batch Fermentation: All nutrients added at once.
◼️ Fed-Batch Fermentation (most common): Nutrients are added gradually, preventing overflow metabolism.
◼️ Continuous Fermentation: Rarely used due to contamination risks.
In summary: Fermentation takes 10–160 hours depending on the process design, producing high concentrations of lysine in the broth.
4. Cell Separation
After fermentation is complete, cells must be separated from the liquid containing lysine. This is achieved by:
◼️ Centrifugation – High-speed separation of biomass.
◼️ Ultrafiltration – Membrane-based separation for cleaner broth.
Efficient cell removal is vital for downstream purification and to avoid contamination in final products.
5. Product Recovery and Purification
The broth undergoes several refining steps:
◼️ Ion Exchange Chromatography: L-lysine binds to cation-exchange resins.
◼️ Elution & Concentration: The amino acid is separated and concentrated.
◼️ Crystallization: L-lysine is crystallized, often as L-lysine hydrochloride.
◼️ Drying & Packaging: Produces a stable, transportable product.
This ensures purity levels above 98–99%, meeting global standards for feed and food-grade lysine.
What is Industrial Production of Amino Acid by Fermentation?
Once clients understand the lysine process, they often ask me about amino acid production more generally. The principle is similar across most amino acids.
Industrial amino acid fermentation is the cultivation of selected microorganisms under controlled conditions, where sugars and nitrogen are converted into specific L-amino acids. These are then recovered and purified for use in food, feed, and pharmaceuticals.
Key Features of the Process
|
Element |
Description |
|
Microbial Strains |
Genetically enhanced bacteria like C. glutamicum, E. coli, or Brevibacterium |
|
Fermentation Medium |
Sugars (glucose, molasses), nitrogen sources, minerals, vitamins |
|
Fermentation Mode |
Mostly fed-batch for high yield |
|
Products |
L-lysine, L-threonine, L-glutamic acid, L-tryptophan, etc. |
Advantages
◼️ Produces only L-amino acids, which are biologically active.
◼️ Uses renewable carbon sources.
◼️ Operates under mild, environmentally friendly conditions.
Compared to chemical synthesis, fermentation is more sustainable, scalable, and selective.
What is the Difference Between Lysine and L-Lysine?
This is a subtle but important question, especially when you talk to nutritionists or regulatory agencies.
Lysine refers to the amino acid in general, while L-lysine is the biologically active form incorporated into proteins by living organisms. The “L” indicates the stereochemistry—the left-handed configuration—recognized by enzymes and ribosomes.
◼️ L-lysine: The natural form, essential for humans and animals.
◼️ D-lysine: Rare, not used in protein synthesis.
◼️ Lysine (without prefix): Usually implies L-lysine in industrial and nutritional contexts.
So, whenever you see “lysine” in feed formulations, supplement labels, or academic literature, you can safely assume it refers to L-lysine.
Why Does This Matter to You?
As someone deeply involved in turnkey engineering for starch deep processing and amino acid fermentation plants, I can tell you that understanding these processes is more than academic. When you plan a new lysine project, you need clarity on:
◼️ Raw Material Flexibility: Can you use corn starch, wheat starch, or molasses based on local supply?
◼️ Process Reliability: How to maintain sterility and reduce contamination risks.
◼️ Downstream Purification: Ensuring food and feed-grade quality.
◼️ Sustainability: Leveraging renewable carbon sources for lower environmental footprint.
If you are evaluating L-lysine investment, knowing the industrial process helps you make better decisions about equipment selection, plant design, and ROI expectations.
Conclusion
Industrial production of L-lysine by fermentation is a shining example of how biotechnology meets large-scale engineering. From strain development to purification, every stage has been refined to maximize yield and reduce costs.
For customers, L-lysine represents not just an amino acid but a strategic input into global food security and animal nutrition. For engineers like me, it’s also a story of innovation—where microbiology, process engineering, and sustainability converge.
If you are planning to enter the lysine business, I encourage you to partner with experienced solution providers who can deliver end-to-end turnkey plants. That’s how you ensure your investment is not only technologically advanced but also future-proof.
拷贝.webp)
